17 Predict Which of the Following Has an Ionic Bond
For example the neutral bromine atom with 35 protons and 35 electrons can gain one electron to provide it with 36 electrons. This results in an anion with 35 protons 36 electrons and a 1 charge.

Ionic Bonding Chemistry Quiz Quizizz
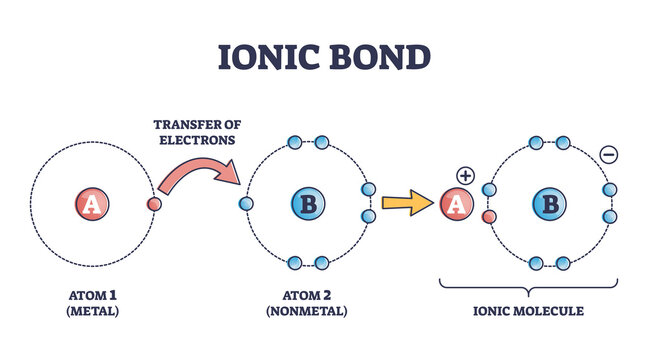
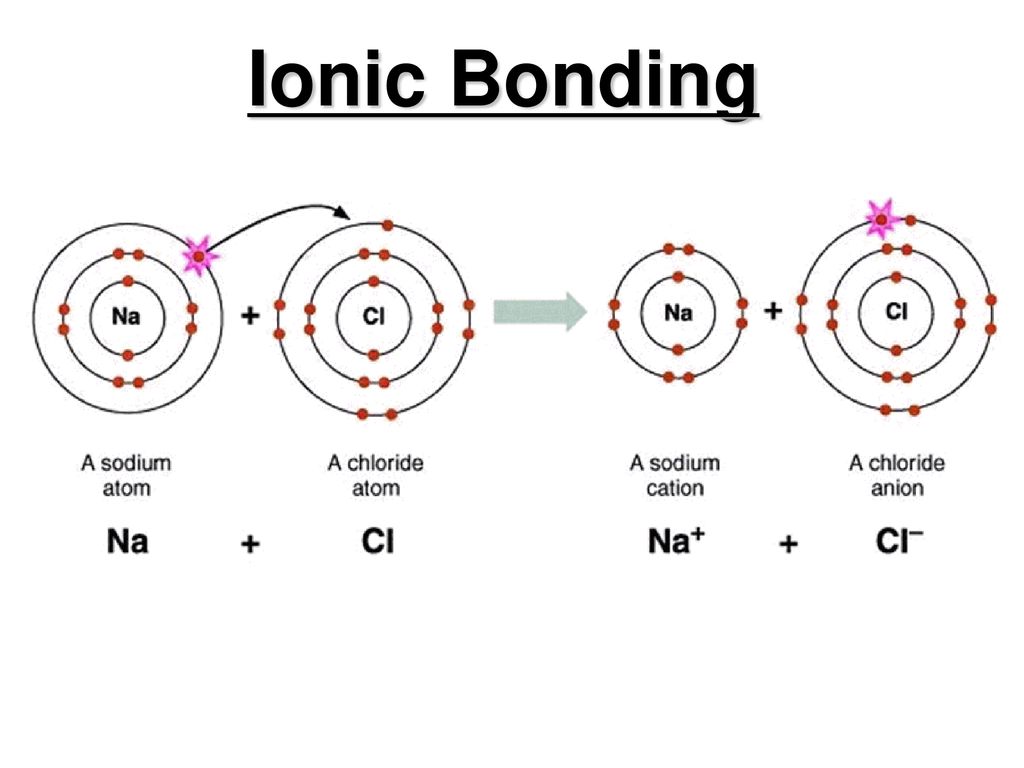
Lets talk about sodium chloride.

. On the left a chlorine atom has 17 electrons. SF6 H2O CO2 NH3 CaO. Al will form a cation with a charge of 3.
For example the H 2 O molecule has four electron pairs in its valence shell. Write the symbol for each ion and name them. Intermolecular Forces and Properties Youll explore how atoms come together to create solids liquids and gases and how subatomic forces govern the properties of everything around you.
8 sigma bonds and 2 pi bonds c. All these methods predicted the FeCax 180 Å and FeCeq 181 Å. Define and describe covalent bonds.
618 Write the name for each of the following ionic compounds. Define and describe ionic bonds. It just depends on the situation so lets take the example of an ionic compound.
Compare ionic covalent and hydrogen bonds in terms of their strength and functions. 11 sigma bonds and 1 pi bond ____ 18. Two lone pairs and two bond pairs.
Atoms of group 16 gain two electrons and form ions with a 2 charge and so on. 10 sigma bonds and 2 pi bonds d. Predict the formula of the ionic compound that forms from magnesium 1 and oxygen.
79 of exam score. The four electron pairs are spread so as to point roughly towards the apices of a tetrahedron. Aluminum and carbon react to form an ionic compound.
Where n A is the oxidation state of A r i is the ionic radius of ion i r A r B by definition and τ 418 indicates perovskite. Carbon will form an anion with a charge of 4. Structure of ionic solids.
Density functional theory DFT is known to accurately predict the harmonic frequencies for single reference systems. Al 3 an aluminum ion. Of the choice below wish one is NOT an ionic compounds PCl5 CrCl6 RbCl PbCl2 NaCl.
It has the same number of. The solubility of ionic compounds in water depends on the type of ions cation and anion that form the compounds. 617 Write the name for each of the following ionic compounds.
Access the answers to hundreds of Chemical bond questions that are. 11 sigma bonds and 2 pi bonds e. VSEPR and bond hybridization.
Which of the following compounds would you expect to be ionic. The solubility of a salt can be predicted by following a set of empirical rules listed below developed based on the observations on many ionic compounds. Given the atomic number predict the number of bonds that an element will form and predict the.
The prediction results are in good agreement with the experimental results. Structure of metals and alloys. For example AgNO 3 is water-soluble but AgCl is water-insoluble.
Predict which forms an anion which forms a cation and the charges of each ion. How many sigma and pi bonds are present in the following molecule. An ionic bond can be very strong.
However the bond angle between the two OH bonds is only 1045 rather than the 1095 of a regular tetrahedron because the two lone pairs whose density or probability. Get help with your Chemical bond homework. Which element is the most electronegative.
8 sigma bonds and 1 pi bond b. 34 Practice Writing Correct Ionic Formulas. Atoms of group 17 gain one electron and form anions with a 1 charge.
Based on number of valence electrons predict the ion an element will form. 44 Practice Writing Correct Ionic Formulas. 22 The electrons in a bond between two iodine atoms I 2 are shared a unequally and the resulting bond is polar b equally and the resulting bond is polar c unequally and the resulting bond is nonpolar d equally and the resulting bond is nonpolar 23 Which of the following solid substances contains positive ions immersed in a sea of.
A high overall accuracy of 92 for the experimental set 94 for a randomly chosen test set of 116 compounds and nearly uniform performance across the five anions evaluated oxides 92 accuracy fluorides 92 chlorides 90. Resonance and formal charge. The ions that we have discussed so far are.
Not only used the COSMO-SAC model to predict the activity coefficient of the solvent but also established a simple model to predict the fugacity coefficients of the four gases CO 2 CH 4 N 2 and H 2 and further calculated the Henrys law constant of CO 2 in the ionic liquid. Distinguish cations from anions. So weve been talking about sodium ions and.
To predict and write correct chemical formulas the key fundamental steps that are required are 1 knowing the charge states of the ions and 2 using basic math to help you determine how many cations and anions are needed to reach a zero charge state 3 writing the chemical forumulas. C 4 a carbide ion. On the left a chlorine atom has 17 electrons.
Chemical Bond Questions and Answers. What is the formula of the compound formed between strontium 2 ions and nitride 3- ions. To predict and write correct chemical formulas the key fundamental steps that are required are 1 knowing the charge states of the ions and 2 using basic math to help you determine how many cations and anions are needed to reach a zero charge state 3 writing the chemical forumulas.
Ionic Bond Images Browse 795 Stock Photos Vectors And Video Adobe Stock
0 Response to "17 Predict Which of the Following Has an Ionic Bond"
Post a Comment